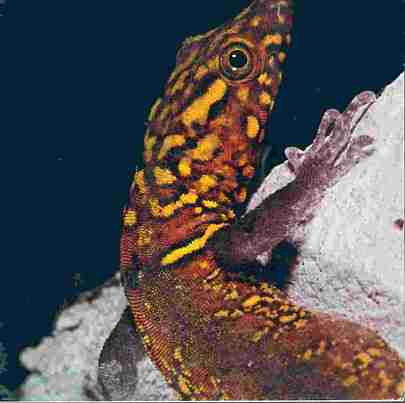
Gonatodes ceceliae, a common inhabitant of the Tamana Caves. (Photo: J.S. Kenny)
Floor plan, environment, and fauna of Tamana caves
By J.S. Kenny
Department of Zoology, U.W.I., St. Augustine, Trinidad.
Reproduced from Living World, Journal of the Trinidad and Tobago Field
Naturalists' Club 1978-1979

Gonatodes ceceliae, a common inhabitant of the Tamana
Caves. (Photo: J.S. Kenny)
INTRODUCTION
Cave systems are of special interest to ecologists because it is relatively easy to measure basic energy inputs and to trace movement of energy through the various elements forming the fauna of the particular system. Trinidad does not have many cave systems and while the few in the Northern Range may be comparatively extensive, they are biologically somewhat uninteresting. There is one cave in Trinidad which has occupied the attention of scientists both at the University of the West Indies and at the Trinidad Regional Virus Laboratory for some time. The Tamana cave system consists of two separate caves of which one has been the site of a study on trophic relationships and energy flow in guano communities. This paper reports on certain related ecological work which had to be done in support of the main studies.
LOCALITIES AND METHODS
Tamana cave is situated on the northern face of Mount Tamana at an elevation of about 240 metres above sea level. In constructing the floor plan, use was made of ten stations and measurements of angles were made with a prismatic compass. Distances between stations and widths of the cave at each station were measured by means of a steel tape. Vertical angles were measured with a bubble sextant. Temperatures were measured with a thermistor chain, while humidity was studied with cobalt thiocyanate paper. Some use was also made of capillary evapori-meters. Faunal collections were made either by direct sampling or after extraction through litter extractors (Berlese funnels).
 Roof in deep part below throat. Bats are mainly Natalus
FORM AND FLOOR PLAN OF THE CAVES The Tamana caves are found in a uniform miocene algal limestone which has been subjected to some intense disturbance. The main cave has been divided into 18 separate sections. (Fig. 1) The highest part of the cave is the walk-in chamber at 1, which is a dimly lit precipitous rock fall and mud slope through which the cave is entered. Adjacent to this, is the boulder chamber which supports a large population of bats. Connecting this outer chamber with the rest of the main cave is a small crawl hole which can be negotiated by people of average build. This crawl hole is also actively used by bats which move freely between the boulder chamber and the deeper parts of the cave. As one passes from the crawl hole into the main cave, on the left is a narrow inclined chamber called the side chamber, which is obviously an old stream course. The crawl hole and this side chamber are separated from the rest of this cave by some vertical ledges. Beneath these ledges, there are two chambers referred to as the round chamber and the long chamber. On the floor of these chambers, there is a narrow stream originating from below ledges in the region of the crawl hole, and both these chambers support extensive roosts of bats particularly the greater spear-nosed bat. The floor of the cave here is uniformly flat and there are heavy deposits of guano. These chambers lead into a comparatively narrow tunnel leading to the chimney area. The general floor topography of the rest of the cave slopes steeply downwards and the vertical dimensions of the passage increase. Two chambers contiguous with the chimney .area may be seen. To the right is the steep passage leading to what may be another old water course, and to the left is the rubble slope produced by the rock debris from a second chimney in the area. This rubble slope leads to a constriction in the chamber called the throat. Beyond the throat, the rest of the cave is divided into a number of chambers separated by ledges but all very much of the same form. Mapping has been extended as far as a chamber called the far deep, but it is unlikely that any further mapping can be entertained, as the physical conditions in the deeper part of the cave are extremely severe both in terms of temperature and humidity. However, the deepest part of the cave has been explored as far as a steep and treacherously slippery guano slope about 100 metres beyond the far deep. It is in this part of the cave that most of the bats live. |
FIGURE I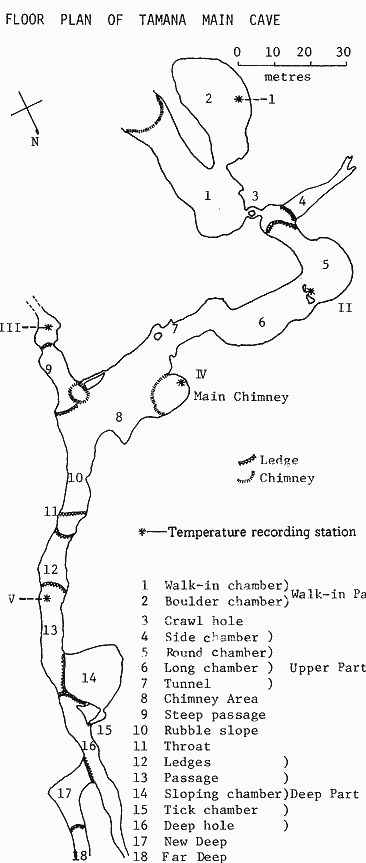 |
 |
CAVE ENVIRONMENT
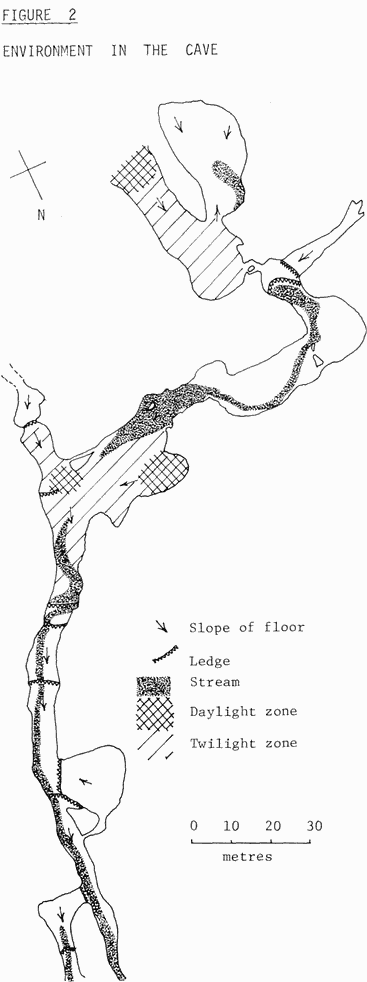
The cave may be divided into distinct zones or regions on the basis of light and into terrestrial and aquatic environments, on the basis of sub-stratum and stream flow. Fig. 2 shows the major divisons of the cave on these bases. At the two chimneys and at the walk-in, there is a daylight zone in which there may be a few green plants. Adjacent to these regions, is a second zone called the twilight zone, in which there is some light reflected from the walls of the cave but not in sufficient quantities to support green plants. The third region of the cave is the region of total darkness and much of the Tamana caves consists of this type of environment.
As the caves are heavily utilised by bats, much of the floor of the cave consists of a superficial guano layer over clay or rock. This is the terrestrial environment of the cave, and this supports a productive community. Passing through the floor of the cave is a small stream. The stream is particularly obvious between the round chamber and the tunnel, where it forms a series of cascades and pools. It, however, disappears underground in the chimney area to re-appear in the rubble slope. Thereafter, the stream may be found superficially throughout the rest of the chambers. The stream supports a fauna consisting mainly of tadpoles in the upper parts of the cave and flatworms in the lower part of the cave.
TEMPERATURE
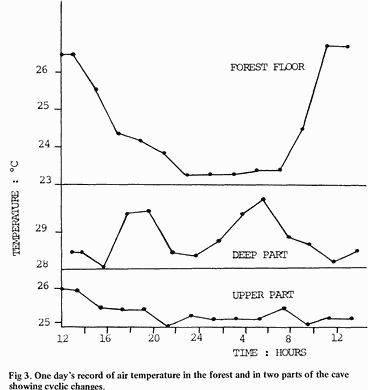
Two prominent temperature features have been discovered at Tamana Cave. The first is a diurnal cycle in air temperature which in the upper part is similar to that in the surrounding forest (Fig 3 top and bottom) but which in the deep part is masked by two peaks (Fig. 3 middle). Moreover, the temperature difference between maximum and minimum is significantly smaller within the cave than outside. Undoubtedly, this is because the cave air is not subjected to sun warming and is furthermore well insulated from contact with the air in the forest. The two peaks in the middle graph (Fig. 3), at 6p.rn.and 6a.m. are caused by mixing of warm air from the roof with cool air on the floor of the cave when the bats move out of and into the deep chamber.
The second temperature feature of the cave environment is the marked thermal stratification demonstrated at all sampling stations. Fig. 4 shows the temperatures recorded between the roof and the floor of the round chamber (5) and in the deep part of the cave (13). Although there are differences between the upper part and the lower part, these profiles are typical of all stations sampled.
The possibility has been
considered that the source of heat in the cave is decomposition of the guano.
However, measurements made within the guano have not shown temperature differences from
air of more than 1°C. The other possibility is that metabolic heat produced by the bats
may in fact prove to be the source of the heat. Preliminary measurements indicate a
thorough mixing of the air mass of the cave associated with the exodus of the bats from
the cave at dusk. This happens daily, and during this time, there is a complete breakdown
of the thermal stratification with uniform temperatures being found from the floor to
the ceiling. After the bats return to the cave, there is a gradual restoration of the
thermal stratification indicating the probability that the two phenomena are linked
causally.
 |
 |
HUMIDITY
Humidity in the caves as determined with cobalt thio-cyanate paper, revealed humidities at all times in excess of 95%, and there appeared to be no difference between dry season and wet season. This method however, revealed slight differences between a forest floor environment in dry season, and cave. The conclusion necessarily, is that the cave environment is constantly at a humidity level near saturation. Hill (1969) did conduct some studies using evapori meters, and was able to establish that even though the humidities in the cave approached saturation, there was still some movement of moisture from the cave floor into the cave air.
VENTILATION
Tamana caves appear to be ventilated by a comparatively simple convection system utilising metabolic heat from the bat communities. In the deep part of the cave, at the throat (11), the movement of air can be demonstrated with smoke markers. The movement of air here on the floor of the throat is downward into the cave, and the air temperature is approximately 24 °C. At the roof of the throat, the movement of air is outward along the ceiling and air temperatures may range from 29°C to 31°C. The flow of air at the throat is continuous throughout daylight hours and during the period of from about 9p.rn.to 5 a.m. The flow definitely ceases between the hours of 6 and 8 p.m.,during which tirne the bats are leaving the cave.
It is concluded that ventilation of the deeper part of the cave is by means of inflow of cool air in the chimney area which flows along the floor of the cave passing through the throat into the deeper part of the cave. The movement of air out of the
cave is along the ceiling of the deep part, upwards towards the small chimney. Ventilation in the upper part of the cave is probably of a similar pattern with inflow at the walk-in chamber. Although there are large numbers of bats in the new deep chamber, no evidence has been produced on mechanisms of ventilation of the deepest chambers. In fact, it is possible that these chambers may have minimal ventilation. There appears to be no thermal stratification in the new deep, with the guano temperatures being approximately the same as the ceiling temperatures — something in the region of about 31°C.
FAUNAE TYPES AND ASSOCIATIONS
Several faunal types and associations have been recognised in the cave system* These are as follows:—
(a) Bat Community
Bats dominate the caves, particularly the deep part. Eleven species have been recorded as permanently roosting in the main cave and one species, Desmodus rotundus, has been recorded roosting occasionally in the adjacent dry cave which has not been studied in detail. The occupation of roosts appears to have varied over the years but there appears to be some stability in general pattern. Pteronotus and Mormoops are definitely confined to the deep part of the cave while Pbyllostomus appears to be more frequently found in the upper part of the cave. Other species such as Natalus and Carollia, seem to be widely distributed throughout the caves. The nectar drinking species, Anoura, is particularly common in the boulder chamber adjacent to the walk-in. It is not clear what is responsible for the distribution of roosts but presumably climatic conditions in the cave may have some influence.
(b) Wall Fauna
Wall fauna consists essentially of arthropods including spiders, cockroaches, crickets, and reduviid bugs. Some of these organisms prey on other arthropods which move on to the walls of the cave.
(c) Guano Fauna
Two distinctive kinds of guano have been recognised in the cave, These include frugiverous guano originating from fruit-eating bats and insectivorous guano, originating from insectivorous bats. The former type of guano tends to be more compact and fluid while the latter tends of be granular, with extensive interstitial spaces. Each guano,, is broken down by separate processes. Frugiverous guano is decomposed and consumed via a bacteria-nematode food chain, whilst insectivorous guano is broken down through a fungi-mite food chain. The guanos of Tamana are heavily populated with blaberine cockroaches that feed either directly on the guano or on fungi growing on the guano.
(d) Stream Fauna
Stream fauna consists essentially of tadpoles of Phyllobates and Planaria. Tadpoles must clearly be bacterial filter feeders and general scavengers while the flatworms are scavengers feeding on dead bats and other organisms.
(e) Aerial Fauna
The aerial fauna of the cave consists of ectoparasitic and free living dipterans as well as lepidopterans and coleopterans. The numers and composition of the aerial fauna varies spatially and temporarally.
(f)Macro Fauna
Supported by the various micro faunal elements, there is a distinctive macro fauna including many vertebrates and some large arthropods. In the chimney area of the cave are invariably found large standing populations of the frog Phyllobates. These frogs are supported largely on emergent insects originating from the guanos and they in turn, are preyed upon by one species of snake Leimodophis. Also found in the cave occasionally, will be other large anurans, particularly Bufo marinus and Leptodactylus podicipinus. The freshwater crab Guinotia, is commonly found in the caves, particularly in the stream, and presumably lives as a scavenger. Occasionally also may be found animals such as the manicou Didelphis and the yellow cribo Pseustes, which presumably enter the caves to feed on bats.
Appendix I lists the more important faunal elements found in the main cave at Tamana. Complete lists of the micro fauna are available in Hill (1969) and Darlington (1970). Generally speaking, the Tamana caves fauna, while rich and exceedingly numerous, shows a close similarity to the fauna of the adjacent forest. While there may be some elements found in the cave not found outside, there is no evidence suggesting that there are any true cavemicolous animals found in Tamana.
REFERENCES:
DARLINGTON, J.P.E.C. 1970. Studies on the Ecology of the Tamana Caves with special reference to cave-dwelling cockroaches. (Ph.D. Thesis - U.W.I)
HILL, S.B. 1969. The Ecology of Bat Guano in Tamana Cave, Trinidad, West Indies. ( Ph.D. Thesis - U.W.I)
APPENDIX I FAUNAL LIST; TAMANA CAVES
Note: While the vertebrate list is complete, only the more obvious macro invertebrates are listed.
VERTEBRATES
Amphibia .. Dendrobatidae Phyllobates trinitatis
.. Leptodactylidae Leptodactylus podicipinus
Leptodactylus boliuianus .. Bufonidae Bufo marinus
Eleutherodactylus urichi .. Ranidae Rana palmipes
Reptilia .. Gekkonidae Gonatodes ceciliae .. Teiidae Bachia trinitatis .. Colubridae Leimadophis reginae Pseustes sulphureus
Mammalia .. Didelphidae Didelphis marsupialis
.. Phyllostomidae Chilonycteris rubiginosa Chilonycteris personata Pteronotus dauyi Phyllostomus hastatus Glossophaga soricina Anoura geoffroyi Carollia perspicillata .. Desmodontidae Desmodus rotundas .. Natalidae Natalus tumidirostris .. Vespertilionidae Myotis nigricans
INVERTEBRATES
Turbellaria undetermined
Crustacea Guinotia garmani Myriapoda Pselliophora cauinicola
Insecta .. Dictyoptera Blaberus giganteus Blaberus atropos Eublaberus posticus Eublaberus distanti .. Orthoptera Lemecella trinitatis .. Dermoptera Spandex percheron .. Hemoptera Phasmatocoris spectrum Panstrongylus geniculatus
Arachinida . .. Pedipalpes Tarantula palmata